JenLab’s non-invasive technology could end surgical biopsies
Professor Karsten König of JenLab – winner of our award for Best Medical Diagnostics Systems Company, 2014 – explains how new laser systems could allow biopsies to be taken without any surgical intervention
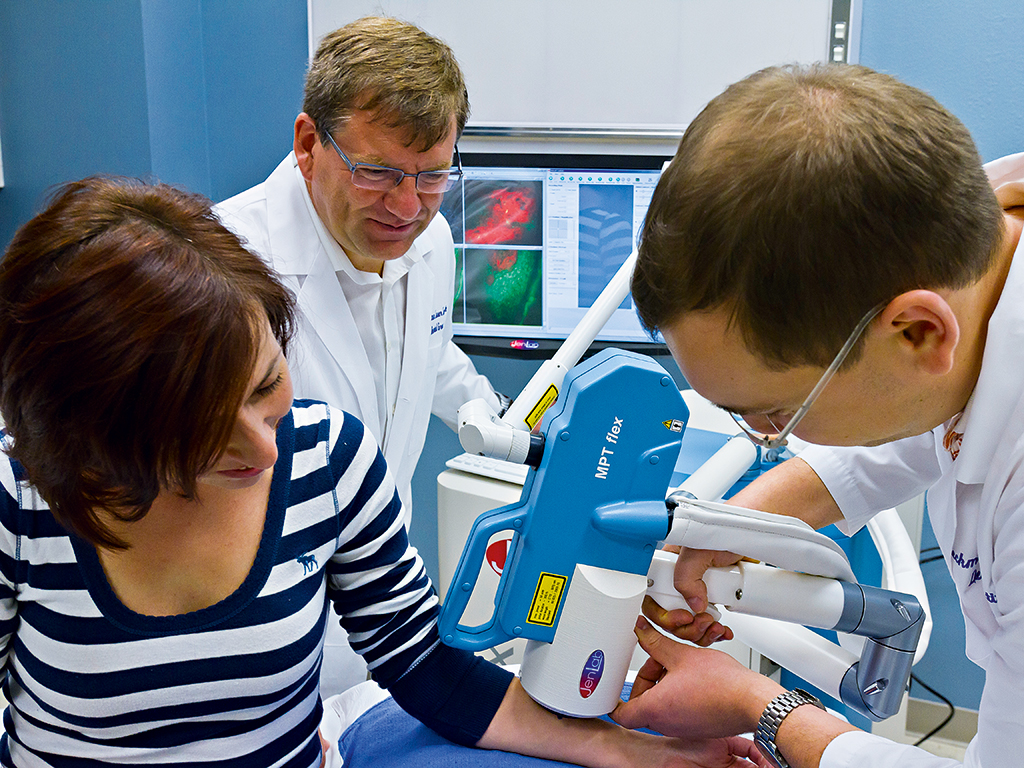
JenLab's clinical multiphoton tomograph MPTflex-CARS displaying an optical section through intact skin
Millions of biopsies are taken every year in hospitals and ambulances around the world for diagnostic purposes. The removed tissue is chemically treated, sliced into 0.01mm-thin histological sections, and stained for evaluation on a subcellular level by pathologists looking through a light microscope. Typically, the patient will receive the diagnosis after several days to one week. This traditional method is costly, time-consuming, laborious, invasive and painful.
It would be a revolutionary step in clinical diagnostics – specifically histopathology – if the tissue in question could be examined with the required high-resolution tomograph non-invasively: meaning non-destructively, without any surgical intervention. This painless examination should occur quickly within seconds or at the most, minutes. It would be ideal if these ‘biopsies’ could be obtained without any labelling while in their native microenvironment, and with information on its chemical composition and physiology, such as its metabolic status.
A major application is the early diagnosis of
skin cancer
Such in vivo histology becomes a reality with the use of femtosecond laser-based multiphoton tomographs. These new clinical imaging tools can provide rapid, scar-free and label-free optical biopsies with chemical fingerprints and superior subcellular submicron resolution. In fact, multiphoton biopsies have at least 1,000 times better resolution than conventional ultrasound, X-ray or MRI images.
How it works
Fermtosecond laser systems have so far been used in refractive eye surgery but not in medical diagnostics. JenLab’s multiphoton tomographs are the first certified medical femtosecond laser diagnostic tools.
The novel tomographs provide a rapid microsopic view into the skin and other tissues by fast-scanning tightly focused near-infrared beams with 80 million laser pulses per second. The mean laser power is equivalent to that of a laser pointer. The beams excite intrinsic biomolecules to emit fluorescence as well as other weak signals that can be detected with single photon sensitivity.
During scanning, high-contrast images of the intratissue architecture appear immediately on the screen of a monitor. The patient can watch their cells, nuclei and organelles immediately on the screen. An optical tissue section takes only seconds: the patient and the doctor can monitor single cancer cells, inflammation sites, the migration of repair cells, the distribution of melanin pigments and even single elastin fibres and the collagen network.
Multiphoton effects were predicted by the PhD student and later Nobel Prize winner Maria Göppert more than 80 years ago. It took 30 years to prove her theory, using lasers that were invented in 1960. A further three decades were required to develop the first two-photon laser microscope in 1990. Nowadays, JenLab’s tomographs make the transition from the lab to the clinical bedside.
Chemical imaging is mainly achieved through a non-linear process called Coherent Anti-Stokes Raman Scattering (CARS), based on molecule vibrations such as with C-H bonds of lipids. The chemical contrast is generated by a titanium-saphhire laser and a photonic crystal fibre that produces an ultrabroadband laser beam.
Practical applications
Besides imaging tissue morphologies and chemical decomposition, functional imaging is feasible due to the fact that biomolecules such as NAD(P)H are involved in cellular metabolism, acting as biosensors. The NAD(P)H level correlates with age. Reactive oxygen species – produced through means such as UV radiation – result in a decrease of NAD(P)H fluorescence, whereas antioxidants provided by healthy food or certain recent anti-ageing products increase autofluorescence. The University of California employs JenLab’s flexible tomograph to study skin physiology and oxygen consumption, while Procter & Gamble uses it to test the next generation of its Olay products, which increase NAD(P)H levels.
A major application is the early diagnosis of skin cancer. Hospitals in Irvine, Brisbane, London, Modena, Nizhny Novgorod and Berlin are employing the recently developed tomograph to detect black skin cancer on a sub-cellular level. Scientists at the Charité in Berlin – the largest hospital in the Eurpean Union – perform in vivo CARS studies on cancer patients, as well as to detect intratissue chemotherapeutics that may cause hand-foot syndrome. Other dermatologists use the tomographs as personalised medical tools to optimise the treatment of dermatitis and actinic keratosis.
JenLab’s tomographs, in combination with microendoscopes, have recently been used in operating theatres during neurosurgery and were able to obtain rapid in situ multiphoton sections from brain tumours. The aim is to obtain direct information on the exact borders of tumours, for precise microsurgery without time-consuming, conventional pathological examinations of physically removed brain biopsies.
The flexible tomograph has also been employed in San Diego at the AntiCancer company to explore potential cancer treatments based on engineered bacteria, as well as the completely non-invasive observation of stem cells within hair follicles.
Journey into space
Major clients of JenLab include cosmetic companies. For the first time they can monitor the in situ accumulation of active compounds and carriers, as well as their interactions with skin components. This is even possible in the forehead and eye regions, and over long periods of time (up to three months). Cosmetic research includes testing the biosafety of sunscreen nanoparticles, which should not penetrate deep to enter blood vessels, as well as to study anti-ageing effects such as the stimulated biosynthesis of collagen. JenLab’s multi photon tomographs are able to define the skin age parameter SAAID by measuring the ratio of elastin to collagen. The SAAID index of a young girl who smokes heavily or regularly visits tanning salons is similar to that of a middle-aged woman.
One of JenLab’s most exciting ongoing multiphoton studies is its collaboration with NASA and the European Space Agency (ESA) in evaluating skin ageing effects in astronauts who are working for half a year at a time on the International Space Station. Skin problems such as dryness, rashes, itchiness, loss of elasticity, thinner skin and slow healing rate are the most commonly described negative impacts on astronauts’ health during space flights. Besides the lack of gravity, astronauts face a significant amount of exposure to extraterrestrial radiation. Furthermore, bioparticles from their own skin tissue – as well as that from other crew members – can cause allergic skin reactions.
The life span of a skin cell is approximately four weeks; meaning skin is renewed once a month – at least on Earth. Scientists involved with ESA-Project Skin B hope to answer the question of how astronauts’ skin regeneration is affected. Your skin will age faster than on Earth, but astronauts may develop more efficient cell regeneration and healing rates upon arriving back home. It is a good opportunity to study the use of skin protective agents containing antioxidants.
Currently, astronauts provide multiphoton biopsies prior to launch and immediately post-flight. For future interplanetary travel, it will be necessary to measure the effects of cosmic rays, biocontamination and microgravity effects while on board. JenLab is working on a device for the next generation: a compact, easy-to-use imaging device for applications both on Earth and in space that can be used to monitor medical risks via optical tissue parameters. Their plan also includes testing this device at high-altitude mountain levels prior to going extraterrestrial.













