Out for blood: the fight against the planet’s deadliest creature
Mosquitoes cause hundreds of thousands of fatalities every year. Technological developments are helping to control the threat, but with funding dwindling, the fight against lethal viruses is far from over
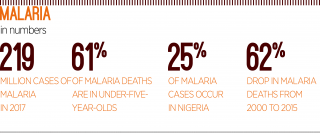
Between 2000 and 2015, deaths caused by malaria fell by around 62 percent, meaning that 6.8 million lives were spared. But this number may start to rise once again, especially as funding for malaria control plateaus
The deadliest creature on the planet is not a great white shark, a starved lion, an enraged hippopotamus or a poisonous snake – it’s the minute mosquito. According to the World Health Organisation (WHO), mosquitoes kill millions of people each year, with malaria – the most deadly mosquito-borne disease – responsible for more than half of these fatalities. The Bill and Melinda Gates Foundation has noted: “Malaria’s economic impact is estimated to cost billions of dollars in lost productivity every year.”
Fortunately, positive developments have been made in recent years to bring the death toll down. Between 2000 and 2015, deaths caused by malaria fell by around 62 percent, meaning that 6.8 million lives were spared. But this number may start to rise once again, especially as funding for malaria control plateaus.
Dr Nathan Rose, Head of Scientific and Regulatory Affairs at Oxitec, a UK-based biotechnology company that develops insect population controls, spoke to The New Economy about the issue: “Over recent years, the number of deaths per year has been decreasing, but that number has now stabilised and may even start to increase again soon because a lot of the tools that have been used for malaria are starting to lose their impact and that includes, unfortunately, some of the main classes of drugs that have been incredibly effective. We’re now starting to see resistance against those, particularly in South-East Asia.”
Yellow fever is spreading and resurfacing in areas where it was thought to be eradicated, including most large urban centres in the American tropics
Dropping like flies
There are thousands of mosquito species, only some of which are vectors for disease. Malaria, for example, is caused by Plasmodium parasites, which are transmitted to humans through the bites of infected Anopheles mosquitoes. Aside from malaria, other mosquito-related viruses have a considerable impact on human health. Several viral diseases are transmitted through the bites of the Aedes aegypti species, including yellow fever, dengue, chikungunya and the Zika virus.
While the Zika virus is not fatal for those bitten, infection during pregnancy can cause severe birth defects. The condition, called microcephaly, causes brain development issues in mild cases and is life-threatening in more severe cases. In 2015, a Zika outbreak that spread across Asia and South America caused a spike in the number of infants born with microcephaly. In fact, in 2016, women in several Latin American countries were warned by government officials to wait until the epidemic was over to conceive.
Dengue, which causes symptoms such as a high fever, painful headaches and vomiting, can be fatal if it’s caught more than once or develops into severe dengue. The spread of the disease, which is a leading cause of death in children in various Latin American and Asian countries, shows no signs of slowing. According to the WHO: “The global incidence of dengue has grown dramatically in recent decades.”
Yellow fever, meanwhile, can be fatal in around 30 percent of cases, Rose said. The WHO states that in sub-Saharan Africa, it is a major public health problem, with 610 million people at risk. It’s also spreading and resurfacing in areas where it was thought to be eradicated, including most large urban centres in the American tropics.
Of these mosquito-borne diseases, only a vaccine for yellow fever exists. “These diseases potentially could impact half the people on the planet because the mosquitoes that transmit them are found so ubiquitously and their range will only spread in coming years as climate change progresses,” Rose told The New Economy. “We’ll see these mosquitoes heading north into Europe and North America and probably further south into other parts of South America, Australia and Africa as well. And so, although these diseases are not as frequently fatal as malaria, they
still have massive impacts.”
Bug off
Thanks to cutting-edge technology, there are new ways to suppress wild populations of Aedes aegypti mosquitoes. The team at Oxitec, for example, breeds male mosquitoes and releases them into the wild to mate with their female counterparts. “All of the female offspring of those matings will die,” said Rose. The process works by adding two new genes to male insects: the first is a fluorescent protein, which helps the team at Oxitec identify their mosquitoes in the wild. The second, and more important, is the self-limiting gene, which produces a protein in females that stops them from developing normally. “It actually stops the female mosquito from developing past the very early larval stages of development,” Rose explained.
The female mosquito will perish before it reaches the reproductive stage of its life cycle, and even before it can fly. “And that’s really important because female mosquitoes are the ones that bite humans and transmit disease. Males don’t. Males just feed on nectar from plants.” The surviving male offspring will continue mating with wild females. Any further female offspring will inherit this self-limiting gene, meaning they too will die. “By doing this, we can actually pretty quickly reduce the population of these disease-transmitting mosquitoes in the wild,” Rose said.
As funding for research has plateaued, fatalities have started creeping up once more
Crucially, the self-limiting protein can be disabled in laboratory settings using an antidote that, along with feed, is added into the water where the larvae develop. “That allows us to produce these mosquitoes in the production facility,” said Rose. “But in the wild, they won’t have access to this antidote, so they won’t be able to survive.”
Oxitec has just completed the first trial of its second-generation mosquitoes – the first used a protein that caused both the males and females to die. The trial took place in the Brazilian municipality of Indaiatuba, just outside of Sao Paulo, in May this year. “We saw up to 96 percent suppression of the wild mosquito population when we released our mosquitoes,
so it was very effective.”
Out of control
Given the success that the project has had so far, it is now expanding. At present, Oxitec is working with the US Environmental Protection Agency (EPA) to conduct its first field demonstrations in the US, which it hopes will take place in 2020. “[The EPA will first] evaluate the safety and the efficacy of the mosquito before we take it into the field, hopefully in Florida,” Rose told The New Economy. “That’s quite important because in the US in recent years they’ve actually had outbreaks of dengue [and] they’ve had locally transmitted Zika, even in Miami. And so there’s a desperate need there for new tools to control these mosquitoes as well.”
Such research is so desperately needed because the Aedes aegypti mosquito is rapidly becoming resistant to the insecticides that are typically used to control mosquito populations. “The mosquitoes have now developed the ability to survive even when those insecticides are sprayed, and so that means the number of tools available to control these mosquitoes is becoming ever more limited,” Rose added.

In fact, according to the WHO’s Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016, there is now widespread resistance to the four most common insecticide classes – pyrethroids, organochlorines, carbamates and organophosphates – throughout the Americas, Africa, South-East Asia, the Western Pacific and Eastern Mediterranean regions. “Since 2010, a total of 68 countries have reported resistance to at least one class of insecticide, with 57 of those countries reporting resistance to two or more classes,” reads the WHO’s website.
Oxitec’s technology could well act as a formidable tool in the fight against mosquito-borne viruses. To this end, the organisation recently received funding from the Bill and Melinda Gates Foundation, the largest private foundation in the world. “We’re developing the same self-limiting technology in Anopheles that can be used in Central America and also in South Asia, [specifically] in India,” said Rose. It will take two to three years of intense research to produce Anopheles mosquitoes with the right set of characteristics to kill female offspring while ensuring males stay healthy enough to be able to mate and to attract mates. “So those are quite significant challenges,” Rose added.
Self-destructive behaviour
Another approach to mosquito control is to use gene-drive technology, which propagates a certain gene in a population to encourage its transmission to offspring. Dr Tony Nolan, a molecular biologist at the Liverpool School of Tropical Medicine, is working on a gene drive using the CRISPR technique. He previously worked on the same research at Imperial College London. He explained: “[It] aims to rapidly transform mosquito populations with genetic elements that either lead to suppression of the mosquito population or to its modification in ways that make the mosquitoes incapable of transmitting the parasite. Gene drives are a type of ‘selfish’ genetic element that bias their own inheritance every generation – every time a mosquito with a copy of the gene drive mates with one that doesn’t, nearly all the offspring inherit the gene drive, meaning that their numbers rapidly increase in the population over a very short time, even if they cause a cost to the population.”
Describing his work thus far, Nolan told The New Economy that his team has recently designed gene drives that can disrupt the fertility of female mosquitoes. This means the gene drives will increase in frequency within a population at the same time that the population’s ability to reproduce is suppressed. “We have shown in the lab that this can lead to invasion of the population and its complete crash very rapidly, even starting from a very low number of released gene-drive mosquitoes,” Nolan added.
The next stage of the project is to test the technology on a much larger scale. Nolan explained that this will take place in bigger enclosures with more realistic settings, which will allow more complex mosquito behaviour to take place. This stage is crucial because understanding behaviours such as mating and egg-laying in the wild is key to successful gene drives. “In the meantime, there are many studies… [into] basic mosquito behaviour and distribution in the wild [being carried out] in order to better understand how this type of technology might work and how best to employ it,” Nolan said.
Such technology does pose risks that must be considered. First, it’s worth noting that there are two main types of gene drives: the first affects the local area only, while the second impacts an entire species. Kevin Esvelt, Director of MIT Media Lab’s Sculpting Evolution group, recommends starting locally to demonstrate how the technology works in various countries and ensure that negative ecological side effects do not transpire. Following these trials, targeting the entire species could theoretically take place.
“Because they spread indefinitely, self-propagating gene-drive systems are much more efficient, but our models predict they will spread on their own if just a handful of carrier organisms escape or are deliberately introduced,” Esvelt told The New Economy. “That’s why self-propagating gene-drive systems should only be constructed or introduced into affected countries once all affected nations have agreed. Crucially, several such systems are likely to be required to overcome pockets of resistance that are likely to evolve… One gene-drive system is extremely unlikely to be enough for eradication, even with the best coordinated effort.”
Esvelt explained that it’s important to gain permission from the authorities before carrying out releases to reduce the risk of a country refusing to cooperate with eradication efforts. Any such blockage would disrupt a project, which could lead to a resurgence in malaria and countless unnecessary deaths. Esvelt added: “Perhaps most importantly, it would set a dangerous precedent for the unilateral use of biotechnologies anticipated to spread across borders without securing permission. As a general rule, scientists should never build constructs that could plausibly spread uncontrollably without the explicit support of all nations
that would be affected.”
No silver bullet
It’s tempting to think that these kinds of solutions could be a panacea for malaria and other mosquito-transmitted diseases, but it’s not so simple. “I think it’s one part of a toolkit that needs to have several different tools in it,” said Rose. “It’s very important to try and get rid of the mosquitoes, but it’s also really important to try and have drugs that you can actually use to deal with the diseases directly where you do have transmission… No single tool is going to be a silver bullet that deals with everything.”
Esvelt added: “Eradicating malaria will require a coordinated effort involving all affected countries, all current prevention and treatment methods and at least one powerful new technology. That technology could be a highly protective and long-lasting vaccine, a long-lasting antimalarial drug or a series of gene-drive systems to alter or suppress the primary mosquito vectors. Vaccine and drug development have proven difficult, whereas functional gene-drive systems have been constructed in malarial mosquitoes.”
The problems caused by the Anopheles and Aedes aegypti loom large. These insects kill millions, cause irreparable health problems and have an enormous economic impact. The war against mosquito-borne diseases cannot be neglected. This is evidenced by the fact that as funding has plateaued, fatalities have started creeping up once more. As such, funding into various avenues must continue, if not increase. Fortunately, gene-editing technology may prove to be so effective it could push the issue over the crucial edge, offering hope to populations around the world.
Some argue that killing off entire species is immoral as we cannot possibly know the harm this could cause to ecosystems. While that may be the case, the extermination of wild mosquito populations would be limited to species that carry diseases that are harmful to humans; there are thousands of others that are not. Neither the Anopheles nor Aedes aegypti species are of any further importance to their environments. In fact, very few mosquitoes are: those found in the Arctic, which do not carry human diseases, are a food source for birds and other insects, but these are not the mosquitoes that will be targeted. It is thanks to the work being carried out by Oxitec, Imperial College London and the Liverpool School of Tropical Medicine that we may soon have a solution that could save countless lives and loosen the grip that mosquitoes have on human health.













